Abstract
Background: Von Willebrand factor (VWF) is a multimeric glycoprotein that plays a central role in primary hemostasis. Synthesized in endothelial cells and megakaryocytes, VWF acts as a molecular bridge that tethers platelets to the site of vascular injury and is also a critical carrier for coagulation factor VIII. Von Willebrand disease (VWD) is the most common inherited bleeding disorder. Type I and type III VWD are most often caused by damaging variants in the VWF gene that affect the synthesis and/or secretion of VWF or the half-life of VWF in plasma. However, around 35% of patients with type I VWD do not have identifiable variants in the VWF gene, suggesting variants in other genes play an important role in VWD. We hypothesized that one mechanism of decrease plasma VWF levels is due to poor secretion of VWF from endothelial cells due to loss of function variants in genes encoding proteins critical to the VWF secretory pathway.
Methods: We performed a genome-wide CRISPR Cas9 knockout screen using the GeCKOv2 pooled library made up of 123,411 guide RNA (sgRNA), targeting nearly each gene in the genome, with six sgRNA per gene. The reporter cell line was HEK293T cells that were stably transfected with a plasmid expressing cDNA for VWF-eGFP in tandem with alpha-one antitrypsin (A1AT)-mCherry. We hypothesized that the knockout of genes required for efficient VWF secretion would cause an accumulation of VWF-eGFP intracellularly and could be identified through flow cytometry-based cell sorting. The reporter cell line was transduced with an index of infection that favored knockout of a single gene per cell. To favor the identification of genes that encode proteins specific for VWF secretion, we first identified cells with normal A1AT-mCherry signal. These cells were then sorted into top and bottom 5%tile bins of intracellular VWF-eGFP. DNA from sorted cells was extracted and the sgRNA of the sorted cells was quantified by next generation sequencing. Each sgRNA targeting gene was then assigned a score based on its frequency in the high versus low VWF-eGFP group. We selected the top 5 genes from the screen and performed single gene knockouts in both HEK293T reporter cells and HUVEC-tert2 endothelial cells that endogenously express VWF. Intracellular VWF signals from HUVEC-tert2 cells was quantified by a polyclonal anti-VWF antibody and like the reporter cells, compared to controls transduced with non-targeting sgRNA.
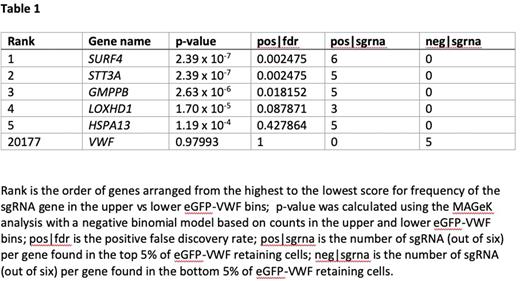
Results: We identified 5 genes for which the sgRNAs were significantly enriched in cells with increased intracellular VWF-eGFP signal. All 5 genes have a plausible role in the secretory pathway and have not been previously associated with VWF secretion (Table 1). The top targeted gene was SURF4, which encodes an endoplasmic reticulum (ER) cargo receptor that recruits specific proteins into vesicles and mediates their transport from the ER to the Golgi. Next, we identified STT3A, encoding a subunit of a protein complex involved in N-linked glycosylation of target proteins in the ER. Ranked third was GMPPB, which encodes a protein involved in the production of N-linked oligosaccharides. Fourth was LOXHD1, which encodes a protein involved in the targeting of proteins to the plasma membrane. Ranked fifth was HSPA13, which encodes heat shock protein family A member 13, a microsome associated protein that has been associated with processing of secreted proteins and degradation of misfolded proteins. To validate these findings, each of the five genes was knocked out individually in both the HEK293T and HUVEC-tert2 cell lines. In the HEK293T cells, flow cytometry results demonstrated increased VWF-eGFP signal in the SURF4 knockout cells but equivocal results for the other four candidate genes compared to cells transduced with non-targeting sgRNA. Interestingly, in the HUVEC-tert2 cells, knocking out each of the top five candidate genes demonstrated increased intracellular VWF retention over baseline, suggesting a potential role for these proteins in the VWF secretory pathway in vivo.
Conclusion: Our study used a non-biased genetic screen to identify potential new trans regulators of plasma VWF levels and may inform the pathogenesis of some forms of Type I and Type III VWD. We identified several candidate genes that may be critical for normal VWF secretion. These genes have not been previously associated with VWF secretion.
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.